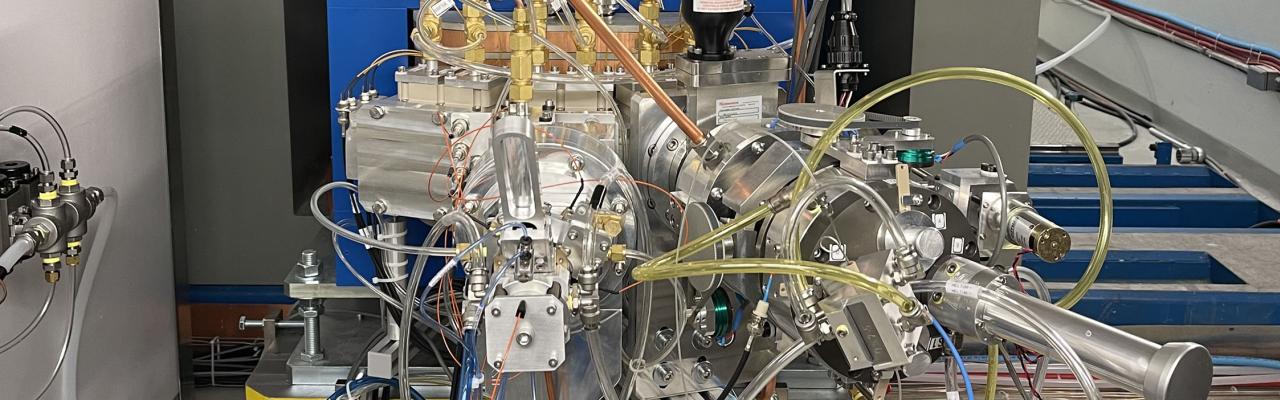
Cyclotron and Radiochemistry Facility
A medical cyclotron (Ebco 19 MeV Dual Beam Cyclotron) is available for production of radiotracers and radiochemistry equipment is available for ligand synthesis.
The cyclotron produces various positron-emitting radiolabeled pharmaceutical drugs that are designed and engineered to complement clinical molecular targets. The preparation of these unique drugs requires a source of radionuclide (such as 11C or 18F) and the tools for subsequent synthesis of the drug incorporating the radionuclide. The cyclotron produces 19.2 MeV protons and 9.5 MeV deuterons in two separate beam lines.
The cyclotron facility consists of a TR-19/9 Cyclotron from Advanced Cyclotron Systems, Inc. (ACSI), negative ion (variable energy up to 19 Mev protons at <150 µA) self-shielded cyclotron with 4 different target ports, housed in a 1 m thick concrete vault. The cyclotron has the basic targetry for the production of F-18, C-11, and N-13. In addition, an external beamline is designed to support a semi-automated solid target system capable of making longer lived pet metallic radionuclides such as I-124, Zr-89, Y-86, Cu-64 and others. The cyclotron is located in the same building as the PET cameras and facilitates the use of short-lived isotopes such as O-15, N-13 and C-11.
The CBIC Radiopharmaceutical Research Core Facility consists of laboratories fully equipped for production and quality control of clinical radiopharmaceuticals and research radio-compounds.
Currently, Citigroup Biomedical Imaging Center (CBIC) at Weill Cornell Medicine holds 15 open IND’s for clinical research protocols including [11C]PIB, [11C]PK11195, [11C]DPA713, [11C]PE2I, [11C]Flumazenil, [11C]Butanol, [11C]Raclopride, [18F]FLT, [18F]FIMSO, [18F]FES, [18F]4-Fluoroglutamine, [18F]FPEB, [68Ga]PSMA-HEBD-CC and [68Ga]DOTATOC. In addition, the radiochemistry laboratory also can provide production services for many other PET tracers per request.
The Radiochemistry Core Facility is fully equipped to perform routine quality control testing for the final drug product release. The production and QC testing labs are closely following cGMP regulatory requirements nsfor the production of PET radiopharmaceuticals according to FDA 21 CFR Part 212 regulations, and/or USP <823> and USP <797> regulations.
Please contact Muc Du for pricing.
Front Desk
Kamaar Lawson
212-746-5889
Center Administrator/Scheduling
Muc Du
212-746-5883
Center Director
Douglas Ballon, Ph.D.
212-746-5679